Objective: The purpose of this study is to report and discuss the use of elective embryo reduction to treat a cesarean scar pregnancy (CSP) combined with intrauterine pregnancy after assisted reproduction, and its clinical outcomes. Material and Methods: Clinical data from six patients who were diagnosed with CSP combined with intrauterine pregnancy were retrospectively collected and analyzed. Four patients underwent elective embryo or fetal reduction following local injection of potassium chloride (reduction group), while the other two patients chose to continue their multiple pregnancies (observation group). Results: All patients were pregnant with multiple chorionic and amniotic fetuses after assisted reproduction. Mild placenta accreta was observed in one patient in the reduction group. In this group, hemorrhage volume during delivery was from 400 to 900 mL, and the average birth weight was 2,776 g. Placenta accreta spectrum occurred in both patients in the observation group, and they gave birth prematurely between 32 and 34 weeks. Conclusion: CSP combined with intrauterine pregnancy was treated using elective embryo or fetal reduction, which may improve maternal and fetal safety. Content: Outcomes of cesarean scar pregnancy with intrauterine pregnancy with or without elective embryo reduction.
Embryo implantation in a cesarean scar, resulting in a cesarean scar pregnancy (CSP), is a special type of ectopic pregnancy [1, 2]. Severe bleeding may occur during early pregnancy owing to the lack of endometrium and myometrium. Most reported CSP cases are single (embryo) CSP [3-7]. Multiple CSP cases are rarer and generally are a result of assisted reproduction [8, 9]. Unlike single and multiple CSP, CSP with intrauterine pregnancy creates a clinical dilemma in which decreasing the risk for CSP and preserving the intrauterine pregnancy are both desirable. Pregnancy termination results in fetal loss and uterine trauma and can cause psychological and physical harm to pregnant women, particularly those who have undergone in vitro fertilization - embryo transfer (IVF-ET). Moreover, the risk for subsequent pregnancies is difficult to assess. Therefore, different from various treatments for single CSP [10-12], it is a challenge for obstetricians and gynecologists to choose an appropriate treatment for CSP with intrauterine pregnancy [13, 14].
In this study, we report six patients who were diagnosed with CSP combined with intrauterine pregnancy at our center. We offered multifetal pregnancy reduction as a new treatment strategy, which was performed on four patients. The other two patients continued their multiple pregnancies without intervention; their clinical outcomes are presented and compared. These results will provide a reference for treating similar clinical conditions.
This was a retrospective study. The patients were diagnosed with CSP combined with intrauterine pregnancy in Xiangya Hospital between January 1st, 2016 and March 1st, 2018. Data on surgical conditions during the perioperative period and the outcomes were collected. The authors had no access to information that could identify individual participants during and after data collection.
Notably, all diagnosed women underwent IVF-ET, and two patients transferred three embryos. The Chinese government issued a regulation in 2003 to control the number of embryos transferred. The regulation stated that no more than three embryos can be transplanted per cycle; up to two embryos can be transplanted during the first cycle in women under the age of 35 years. In our cases, the two patients who received three embryos had undergone more than two failed embryo transfer cycles, and their ages were more than 35 years, respectively. Therefore, according to the regulations at that time, they were allowed to receive three embryos each. Both patients had a strong desire to transfer three embryos due to their previous transfer failure. They had been fully informed of the risks and benefits of multiple (three) embryo transfer and provided informed consent. Based on these regulations and the patients’ strong desire, three embryos were transferred to these patients in 2016 and 2017. This practice has been updated according to the latest Chinese Expert Consensus on Numbers of Embryos Transferred published in 2018 (in Chinese) [15]. The consensus focused on reducing the number of multiple pregnancies by controlling the number of embryos transferred per cycle to no more than two and implementing the strategy of selective single embryo transfer. Because this was a retrospective study that focused on the outcomes of embryo/fetal reduction, patients who had previously (before 2018) received treatment were included.
All ectopic embryos met the CSP diagnostic criteria according to the Godin standard [16]. All patients were diagnosed based on a history of cesarean delivery, elevated serum levels of β-human chorionic gonadotrophin (β-hCG), and ultrasound characteristics including an empty cervical canal, a myometrial defect between the sac and the bladder wall, and a gestational sac located at the anterior part of the uterine isthmus. Unlike single CSP, CSP combined with intrauterine pregnancy should contain live intrauterine embryo(s), which was validated by ultrasound.
All patients who fulfilled the diagnostic criteria were included in this study. The diagnosis, therapy, and follow-up of all patients were performed at the Xiangya Hospital Central South University. Both embryonic/fetal heart activities of the ectopic embryo/fetus and intrauterine embryo/fetus were demonstrated at the time of ultrasound; a clearly stated desire from the patient for continuing the pregnancy after evidence-based counseling describing the options for continuing or terminating the pregnancy. Patients with single CSP, cervical pregnancy, uterus isthmus gestation, inevitable abortion, incomplete abortion, and gestational trophoblastic disease were excluded.
This retrospective study only collected medical data and could not intervene in the medical procedure. The details of the treatment procedures are described here. All patients were fully informed of the risks and benefits of all treatment options. The three therapeutic strategies were continuing with the multiple pregnancies, terminating the multiple pregnancies, and continuing the intrauterine embryo(s) while simultaneously terminating the ectopic embryo(s). All patients made their own treatment decisions after detailed discussions with their doctors. Based on evidence at that time, different treatments had different risks. These options were not given equally. The risk of continuing a pregnancy (particularly the risks of hemorrhage, placenta percreta, and hysterectomy) was emphasized.
Essential obstetric practices were performed throughout the pregnancy for the patients who chose to continue their multiple pregnancies. Abdominal pain, placental attachment, the possibility of massive hemorrhage, uterine rupture, and placenta increta were assessed. Termination occurred if necessary. Suction curettage, surgical resection, and hysteroscopic excision were considered for patients who chose to terminate their pregnancies. The basic principle was to remove the focus while preserving fertility.
Elective reduction was applied for patient who chose to continue the intrauterine embryo(s)/fetus(es) and terminate the ectopic embryo(s)/fetus(es). Due to the (bleeding) risks for CSP, this procedure should be performed as soon as possible after the treatment choice is made by the patient. Our cases had different gestational weeks when they saw a doctor and made treatment choices. Therefore, the gestational ages for intervention were from 7 to 16 weeks. Specifically, potassium chloride (KCl) was injected locally into the ectopic embryo/fetus, which had small impact on the intrauterine embryo(s)/fetus(es). Follow-up consisted of ultrasound examinations and routine obstetric examinations. Weekly serum levels of β-hCG were not assessed owing to the live intrauterine embryo(s)/fetus(es).
Local injection of KCl was used as an elective embryo/fetal reduction treatment. The patient was placed in the dorsal lithotomy position. Preoperative preparations were completed. Ultrasonographic scanning was used to determine the location of the embryos in the uterine cavity and cesarean scar of first-trimester patients. The CSP was chosen as the target embryo. Fine-needle puncture (22G) was performed using ultrasound to ensure accurate needle placement. Next, we inserted a thin needle directly into the cardiac position and aspirated a small volume of fluid or blood along the line. It was necessary to turn the needle to confirm injection into the cardiac position of the embryo. Then, we injected 10% KCl (2-10 mL) through the needle. The dosage was related to gestational age. The heartbeat disappeared on ultrasound. Reconfirmation was performed via ultrasound after 5-10 minutes (Figures 1 and 2).
 Figure 1.
Figure 1.— A patient from the intervention group prior to operation. Ultrasonic images demonstrating ectopic and intrauterine pregnancies, showing two gestational sacs (G1, G2). One of the sacs (G1) is within the anterior wall of the uterus, surrounded by myometrium and scar tissue. The abundant peritrophoblastic flow is clearly depicted. G, gestational sac; CX, cervix; AS, ascites.
 Figure 2.
Figure 2.— A patient from the intervention group after the operation. Ultrasonic images demonstrating ectopic pregnancies. After the operation, the fetal heartbeats disappeared and the surrounding flow dropped, which was checked by ultrasound.
Transabdominal ultrasound was used as a guide for patients in the second trimester. We performed aforementioned puncture and injection steps when the fetus was still. KCl was injected into the cardiac region or the brain. If the fetal heart recovered, the injection step was performed repeatedly until the heartbeat disappeared from ultrasound. The absence of an ectopic fetal heartbeat indicated a successful KCl injection and thus completion of this step. The intrauterine fetus was also checked for a normal heartbeat.
Six patients met the criteria during the study period. They all received IVF-ET: five had twins and one had triplets. All patients were pregnant with multiple chorionic and amniotic fetuses. The average age of the patients was 35.67 (range 31-39) years, with average gravidity of 4.17 (median 4.5, range 3-5) and parity of 1.33 (median 1, range 1-2). The time between the previous cesarean delivery and the index pregnancy was 2-14 (average 9.5) years. Five patients (5/6, 83.33%) had a history of vaginal bleeding, and one had visited a doctor without any positive symptoms (Table 1).
| Case No. | Age (y) | IVF-ET | Survived/ Transferred embryos | Gravida | Para | No. of previous cesarean deliveries | CS time (y) | GAI (weeks) | Abdominal pain | Vaginal bleeding |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | Yes | 2/2 | 4 | 1 | 1 | 4 | 8 + 5 | Negative | Positive |
| 2 | 39 | Yes | 2/2 | 5 | 1 | 1 | 13 | 7 + 6 | Negative | Positive |
| 3 | 39 | Yes | 2/3 | 3 | 1 | 1 | 11 | - | Negative | Positive |
| 4 | 37 | Yes | 3/3 | 5 | 2 | 2 | 14 | 15 + 3 | Negative | Negative |
| 5 | 35 | Yes | 2/2 | 5 | 2 | 2 | 13 | 16 + 4 | Negative | Positive |
| 6 | 33 | Yes | 2/2 | 3 | 1 | 1 | 2 | - | Negative | Positive |
Note: IVF-ET, in vitro fertilization-embryo transform; CS time, time between previous cesarean delivery and index pregnancy; GAI, gestational age at the time of intervention (gestational age of the ectopic pregnancy at the time of termination).
Three therapeutic strategies were offered to the patients. None of the patients chose to terminate their multiple pregnancies. Four patients received elective embryo reduction as a treatment, whereas the other two patients chose to continue their multiple pregnancies with more intensive outpatient visits. All patients were followed until delivery or termination of the pregnancy.
Therefore, the patients were separated into two groups: the reduction group (n = 4), composed of patients who received elective embryo reduction within 7 to 17 gestational weeks (average, 12 + 1 weeks); and the observation group (n = 2), composed of patients who chose to continue multiple pregnancies and did not receive an intervention after providing informed consent.
Table 2 presents the data on CSP surgical conditions combined with intrauterine pregnancies. No obvious abnormalities were detected, including abruption or bleeding, during intrauterine gestation in any of the patients.
| Case No. | GAI (weeks) | Embryos | Methods | Operative approach | 10% KCl (mL) | Fetal heart beat | Fetal heart beat after operation (10 min/24h) | Postoperative bleeding |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 + 5 | Dichorionic Diamniotic | Local KCl | Abdomen | 2 | Positive | Negative / Negative | Negative |
| 2 | 7 + 6 | Dichorionic Diamniotic | Local KCl | Transvaginal | 2 | Positive | Negative / Negative | Negative |
| 3 | - | Dichorionic Diamniotic | Observation | - | - | Positive | - | - |
| 4 | 15 + 3 | Trichorionic Triamniotic | Local KCl | Abdomen | 10 | Positive | Negative / Negative | Negative |
| 5 | 16 + 4 | Dichorionic Diamniotic | Local KCl | Abdomen | 10 | Positive | Negative / Negative | Negative |
| 6 | - | Dichorionic Diamniotic | Observation | - | - | Positive | - | - |
Note: GAI, gestational age at the time of intervention (gestational age of the ectopic pregnancy at the time of termination); local KCl, local injection of potassium chloride.
1) Reduction group
In the reduction group, the heartbeats of the target embryos were checked by color Doppler ultrasound at 10 min and 24 h after KCl injection, but disappeared (0/4) (Table 2). Because Cases 4 and 5 had older gestational age, their KCl doses were adjusted. No infection or abortion occurred in these four cases. No obvious change in the hemogram or coagulation index was detected in the third trimester during the monitoring period. Threatened preterm occurred in Case 2 but was cured by symptomatic treatments. Preterm labor occurred at the gestational age of 33 weeks in Case 5, and a cesarean section was performed immediately. Mild placenta accreta was observed in Case 1 of the reduction group. No placental percreta was noted in this group.
All patients in this group underwent a cesarean delivery. The Apgar scores were 8 to 10 (1 min) and 9 to 10 (5 min) for the newborns, and the average birth weight was 2,776.00 g (2,040.00-3,250.00 g). Hemorrhage volume during delivery was 400-900 mL (average 537.50 mL) (Table 3 and Table 4). No fetal abnormalities were observed.
| Case No. | GAI (weeks) | Methods (intervention) | PI | Intrauterine abortion | GAD (weeks) | TPL | Delivery | Number of live births | Apgar score | Hemorrhage of delivery (mL) | Neonatal weight (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 min | 5 min | |||||||||||
| 1 | 8 + 5 | Local KCl | Negative | Negative | 38 + 4 | Negative | C | 1 (IU) | 10 | 10 | 450 | 3,250 |
| 2 | 7 + 6 | Local KCl | Negative | Negative | 38 + 1 | Positive | C | 1 (IU) | 10 | 10 | 400 | 3,150 |
| 3 | - | Observation | - | - | 33 + 6 | Positive | C | 2 (IU, CS) | 9, 10 | 10,10 | 8,000 | 2,300; 1,960 |
| 4 | 15 + 3 | Local KCl | Negative | Negative | 37 + 0 | Negative | C | 2 (IU) | 10, 10 | 10,10 | 900 | 2,040; 3,000 |
| 5 | 16 + 4 | Local KCl | Negative | Negative | 33 + 3 | Positive | C | 1 (IU) | 8 | 9 | 400 | 2,440 |
| 6 | - | Observation | - | - | 32 + 1 | Positive | C | 2 (IU, CS) | 9, 10 | 9,10 | 1,100 | 1,700; 1,350 |
Note: Neonatal weight, the order of neonatal weight and Apgar score are consistent. GAI, gestational age at the time of intervention (gestational age of the ectopic pregnancy at the time of termination); local KCl, local injection of potassium chloride; PI, postoperative (intervention) infection; GAD; gestational age of delivery; TPL, threatened premature labor; KCl, potassium chloride; C, cesarean section; IU, intrauterine fetus; CS, cesarean scar fetus (at the time of diagnosis).
| Group | N | Postoperative abortion | Mean gestational age of delivery (weeks) | Preterm birth rate | Delivery | Apgar score | Mean hemorrhage of delivery (mL) | Mean neonatal weight (g) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 min | 5 min | ||||||||
| Reduction (local KCl) | 4 | Negative | 36 + 5.50 | 1/4 | CS | 9.60 | 9.80 | 537.50 | 2,776.00 |
| Observation | 2 | Negative | 33 + 0.00 | 2/2 | CS | 9.50 | 9.75 | 4,550.00 | 1,827.50 |
Note: local KCl, local injection of potassium chloride; CS, cesarean section.
2) Observation group
All patients in the observation group were informed of the risks, including those of selective reduction and continuation of the multiple pregnancies (risks of hemorrhage, premature delivery, placental percreta, and hysterectomy). These patients ultimately chose to continue their pregnancies with more intensive outpatient visits. The follow-up included routine screening for signs and symptoms of pregnancy, and assessments of fetal condition.
Placenta accreta spectrum occurred in both patients (Figure 3), and they gave birth prematurely between 32 and 33 weeks. Uncontrolled bleeding was the major challenge associated with the cesarean section. During the operation, the internal iliac artery was ligated, and local suturing was used to control bleeding. One woman (Case 6) successfully preserved her uterus and was diagnosed with placental increta. After using the hemostatic procedures described above, a subtotal hysterectomy was performed in Case 3 due to severe bleeding (8,000 mL) caused by placenta percreta. Then the placenta penetrated the myometrium and the serosal surface was confirmed.
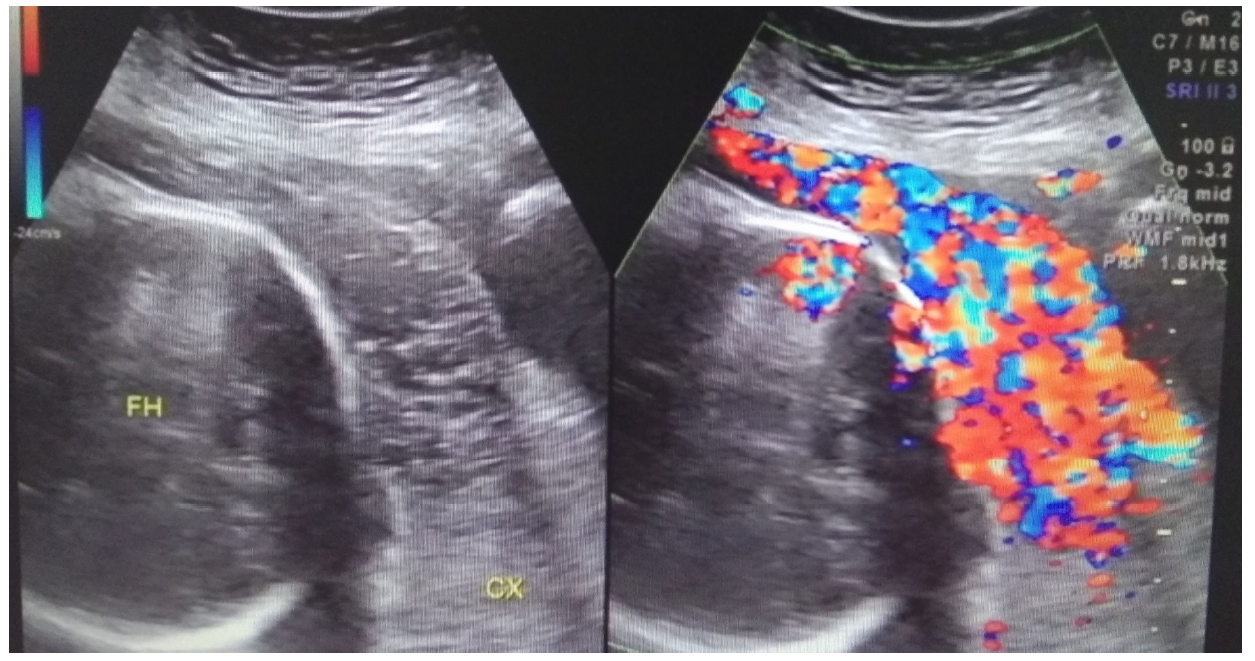 Figure 3.
Figure 3.— A patient from the observation group during the third trimester. Abundant blood flow signals are apparent at the area of the placenta on color Doppler flow imaging. FH, fetal head; CX, cervix.
The Apgar scores were 9 to 10 (1 min) and 9 to 10 (5 min) for the newborns. Due to the premature delivery, the average birth weight was 1,827.50 g (1,350.00-2,300.00 g). No fetal abnormalities were observed (Table 3 and Table 4).
Treatment options for CSP should be based on the patient’s condition and the surgeon’s skill [3, 17]. These treatments include surgical removal, hysteroscopic resection, and suction curettage [4-7, 18-21]. Among them, a local KCl injection has been reportedly used to successfully treat CSP [20, 22]. Injections of KCl (2-4 mL) were performed for two singleton CSP patients around 6 gestational weeks, which was similar to our treatment [20]. A unified CSP treatment program has not been established due to the low incidence and lack of sufficient evidence. In comparison, CSP combined with intrauterine pregnancy is rarer. The biggest challenge is the delicate balance between termination and continuation of pregnancy. Many treatments for singleton CSP cannot be performed, such as systemic methotrexate, when an intrauterine embryo needs to be preserved.
Most publications on CSP combined with intrauterine pregnancy are single case reports on patients who had received IVF-ET [13, 14, 22, 23]. This suggests the possibility that CSP combined with intrauterine pregnancy is a complication of assisted reproduction. Various treatments include conservative treatment, fetal reduction, laparoscopic resection, and suction curettage [24-26]. Despite the small number of cases, many studies have used local injections to reduce the number of fetuses [24, 27, 28].
In this study, our concerns were the local extrusion force from the needles and the possibility of drug leakage, which may affect intrauterine fetal. As an operation, postoperative infection and subsequent miscarriage should also receive attention. Therefore, intrauterine fetal heartbeat, infection, and miscarriage were reported in the results. In our study, a normal intrauterine fetal heartbeat was observed after operation, with no apparent abnormalities. During subsequent monitoring, no obstetric infections or miscarriage were observed. However, threatened preterm occurred in one patient. Similar complications have been observed in selective reduction for multiple pregnancies [29], including pregnancy loss and preterm delivery.
In previous studies on local KCl injections, the interventions were performed primarily at 6-8 gestational weeks [24, 30, 31]; only one study intervened at 16 weeks [32]. Our findings are consistent with these studies and suggest that a local KCl injection could be used during the second trimester. Complications of this treatment include vaginal bleeding and premature delivery [32]. One study used a local methotrexate injection as a treatment [28]. The biggest risk may be the side effects of the drug, including abnormal embryonic development [28, 33]. This study did not detect any obvious intrauterine fetal abnormalities. With regarding to these two similar injections, many studies have used KCl and the safety of KCl has been assessed, however the use of methotrexate may not be fully evaluated yet due to the small number of reported cases.
Surgical treatments, such as laparoscopic or hysteroscopic resection and suction curettage, have been performed early in pregnancy (about 7-8 weeks) and have been found to successfully retain the intrauterine fetus [14, 25, 26, 34]. Anesthesia, uterine trauma, and bleeding risk for CSP should be considered before the operation. Importantly, this may require more surgical skill from the obstetrician. Our cases were informed about the treatment options (benefits and risks), including laparoscopic or hysteroscopic resection and suction curettage for CSP. Considering that surgery may have a greater impact on the uterus, these patients chose the KCl treatment.
After the intervention, most cases were delivered within 30-36 weeks (premature delivery) [24, 31, 35]. One of our patients in the intervention group experienced a premature delivery, and the remainders were delivered at full term. This suggests that full-term delivery is still possible after an intervention in the second trimester [32]. The gestational ages of the observation group were 32-33 weeks, indicating a high risk for preterm birth in CSP patients.
Due to the high risks associated with CSP, once embryo/fetal reduction is determined, the intervention could be performed as soon as possible, which could benefit the intrauterine embryo/fetus and reduce postoperative complications [18]. CSP combined with intrauterine pregnancy is generally diagnosed at 5-7 weeks, so the gestational weeks of intervention are at least 5-7 weeks [30]. The gestational age of the intervention depends on when the patient visits, the time it takes for diagnosis, and the time it takes for a pregnant woman to make a final decision. Due to these factors, two of our cases ultimately accepted an intervention during the second trimester.
Preterm birth (32-33 weeks), massive hemorrhage, and hysterectomy (was related to placenta accreta spectrum) occurred in the observation group. These are adverse events of CSP that develop in the third trimester. Therefore, the expectant CSP treatment should be strictly managed. We fully informed the pregnant women about the risks of the expectant treatment, particularly the heavy hemorrhage, premature delivery, and placenta accreta spectrum. However, possibly because of the difficulty of each pregnancy (having been accomplished via IVF-ET) and uncertainty about subsequent pregnancies, both women insisted on continuing their pregnancies. In the follow-up, we strictly managed these cases as high-risk pregnancies but due to severe intraoperative bleeding, there was still a case of hysterectomy, suggesting that CSP combined with intrauterine pregnancy may develop into placenta accreta spectrum. There is a high risk for preterm birth and massive hemorrhage resulting from placenta accreta spectrum. The subsequent possibility of hysterectomy is also a serious threat. The higher risk for premature birth, as well as lower birth weight, should also be considered. One study reported that a significant proportion of CSP pregnancies progress to term; thus, terminating the pregnancy may not be the only therapeutic option [36]. This suggests that expectant CSP treatment may need to be reevaluated [36]. As CSP and CSP combined with intrauterine pregnancy occur in different situations (such as IVF-ET), it should be encouraged to report more CSP combined with intrauterine pregnancy cases to fully assess the treatment and provide more comprehensive information. In short, the high risk for expectant treatment and the patient’s desires need to be fully evaluated [18, 36, 37].
Most related literatures are case reports; in this study, we compared the outcomes of treatment methods in a homogenous population, which may provide a better reference for evaluating the effectiveness and safety of these methods. However, our study had some limitations and potential biases that should be discussed. First, as a retrospective study, some patients lacked long-term follow-up data. The sample size was limited because it is a rare disease. Another limitation is that we only evaluated local injection of KCl. More studies evaluate various methods will help evaluate the best treatments for this disease. In addition, this retrospective hospital-based study may involve recall bias and selection bias. To minimize the recall bias, we extracted objective data from medical records. However, the selection/admission bias cannot be controlled. Therefore, our results are likely to reflect the outcomes of the population that tends to visit hospital for consultation and treatment.
In conclusion, CSP combined with intrauterine pregnancy can be treated by elective embryo reduction to improve maternal and fetal safety.
The study protocol was approved by the Medical Ethics Committee of the Xiangya Hospital Central South University (201803284). Informed consent was obtained from all patients before data collection. All procedures in studies involving human participants were performed in accordance with the ethical standards of the Institutional Research Committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The authors thank the ultrasound doctors (Kuifang Shen, Lijuan Pan, Hui Yang and Yimei Fu) from ultrasound group of obstetrics department of Xiangya Hospital Central South University for their assistance in interpreting the clinical relevance of the ultrasound reports. This work was supported by the Hunan Provincial Innovation Foundation for Postgraduate (CX2017B067), the National Twelfth-Five Year Research and Development Program of China (2014BAI05B05), the Science and Technology Project of Hunan Province (2017SK2151, 2017SK1033), the National Natural Science Foundation of China (81974236, 81903696, 81571516), the National Key Research and Development Program of China (2016YFC1000206), and the Major Scientific and Technological Projects for Collaborative Prevention and Control of Birth Defects in Hunan Province (2019SK1010).
The authors declare no conflict of interest.
