- Academic Editor
-
-
-
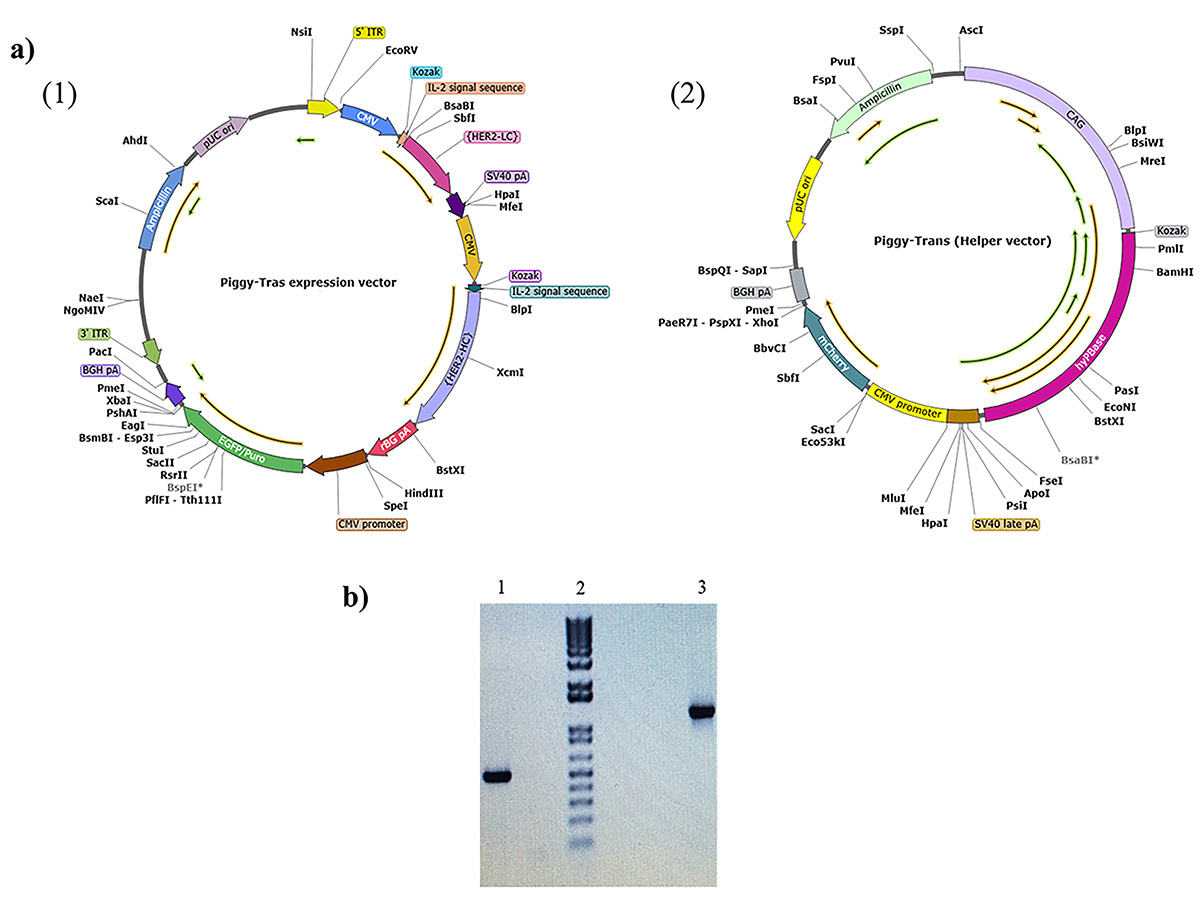
Background: Trastuzumab (Herceptin®) is currently the main treatment option for breast cancer patients that overexpress the human epidermal growth factor receptor 2 (HER2). This antibody binds specifically to HER2, blocks cancer cell growth, and promotes effective cell death. In the present study, we sought to develop a robust and efficient process for the development of a stable Chinese hamster ovary (CHO) cell line with high trastuzumab expression and production. Methods: We adapted a process that combines transposon system-based vector construction, suspension cell culture, and a high selection process. The latter, involved enhanced green fluorescent protein (eGFP) expression, fluorescence-activated cell sorting (FACS), and semi-solid methylcellulose media. Results: The construction of trastuzumab as a humanized monoclonal antibody was achieved by subcloning the synthesized light and heavy chain sequences into a suitable piggyBac expression vector. The optimized piggyBac vector used for the expression of trastuzumab in CHO cells resulted in the production of trastuzumab and reached 4.24 g/L in the T1A7 clone after a 7-day batch culture. The T1A7 clone was selected after screening over 1500 clones. Conclusions: The current simple workflow ensures strict monoclonality and relatively high production of trastuzumab. This workflow could potentially be implemented in Research and Development (R&D) laboratories, including in developing countries for the production of recombinant monoclonal antibodies in a cost-effective manner.