- Academic Editor
-
-
-
†These authors contributed equally.
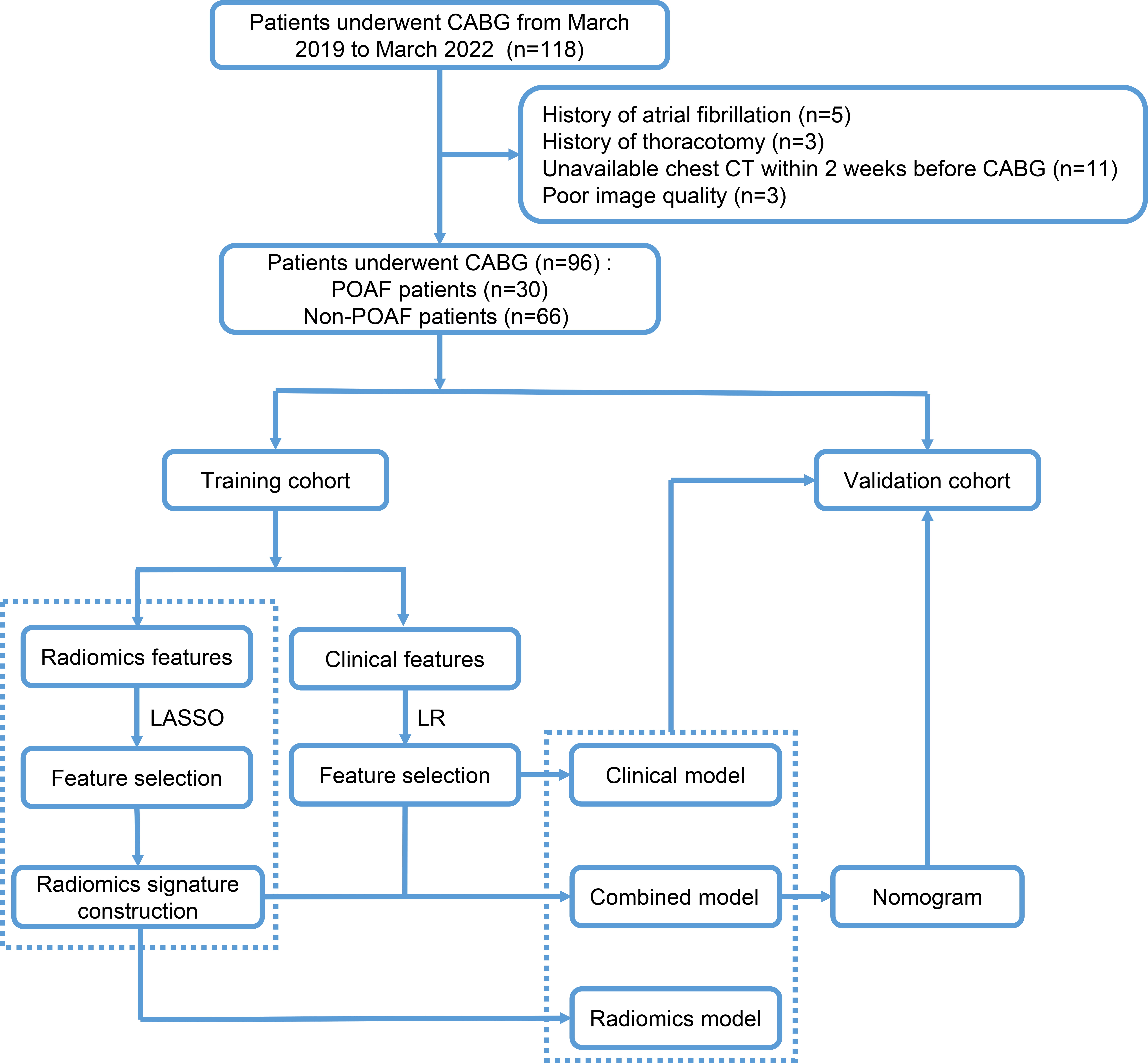
Background: Postoperative new atrial fibrillation (POAF) is a commonly observed complication after off-pump coronary artery bypass surgery (OPCABG), and models based on radiomics features of epicardial adipose tissue (EAT) on non-enhanced computer tomography (CT) to predict the occurrence of POAF after OPCABG remains unclear. This study aims to establish and validate models based on radiomics signature to predict POAF after OPCABG. Methods: Clinical characteristics, radiomics signature and features of non-enhanced CT images of 96 patients who underwent OPCABG were collected. The participants were divided into a training and a validation cohort randomly, with a ratio of 7:3. Clinical characteristics and EAT CT features with statistical significance in the multivariate logistic regression analysis were utilized to build the clinical model. The least absolute shrinkage and selection operator (LASSO) algorithm was used to identify significant radiomics features to establish the radiomics model. The combined model was constructed by integrating the clinical and radiomics models. Results: The area under the curve (AUC) of the clinical model in the training and validation cohorts were 0.761 (95% CI: 0.634–0.888) and 0.797 (95% CI: 0.587–1.000), respectively. The radiomics model showed better discrimination ability than the clinical model, with AUC of 0.884 (95% CI: 0.806–0.961) and 0.891 (95% CI: 0.772–1.000) respectively for the training and the validation cohort. The combined model performed best and exhibited the best predictive ability among the three models, with AUC of 0.922 (95% CI: 0.853–0.990) in the training cohort and 0.913 (95% CI: 0.798–1.000) in the validation cohort. The calibration curve demonstrated strong concordance between the predicted and actual observations in both cohorts. Furthermore, the Hosmer-Lemeshow test yielded p value of 0.241 and 0.277 for the training and validation cohorts, respectively, indicating satisfactory calibration. Conclusions: The superior performance of the combined model suggests that integrating of clinical characteristics, radiomics signature and features on non-enhanced CT images of EAT may enhance the accuracy of predicting POAF after OPCABG.