- Academic Editor
-
-
-
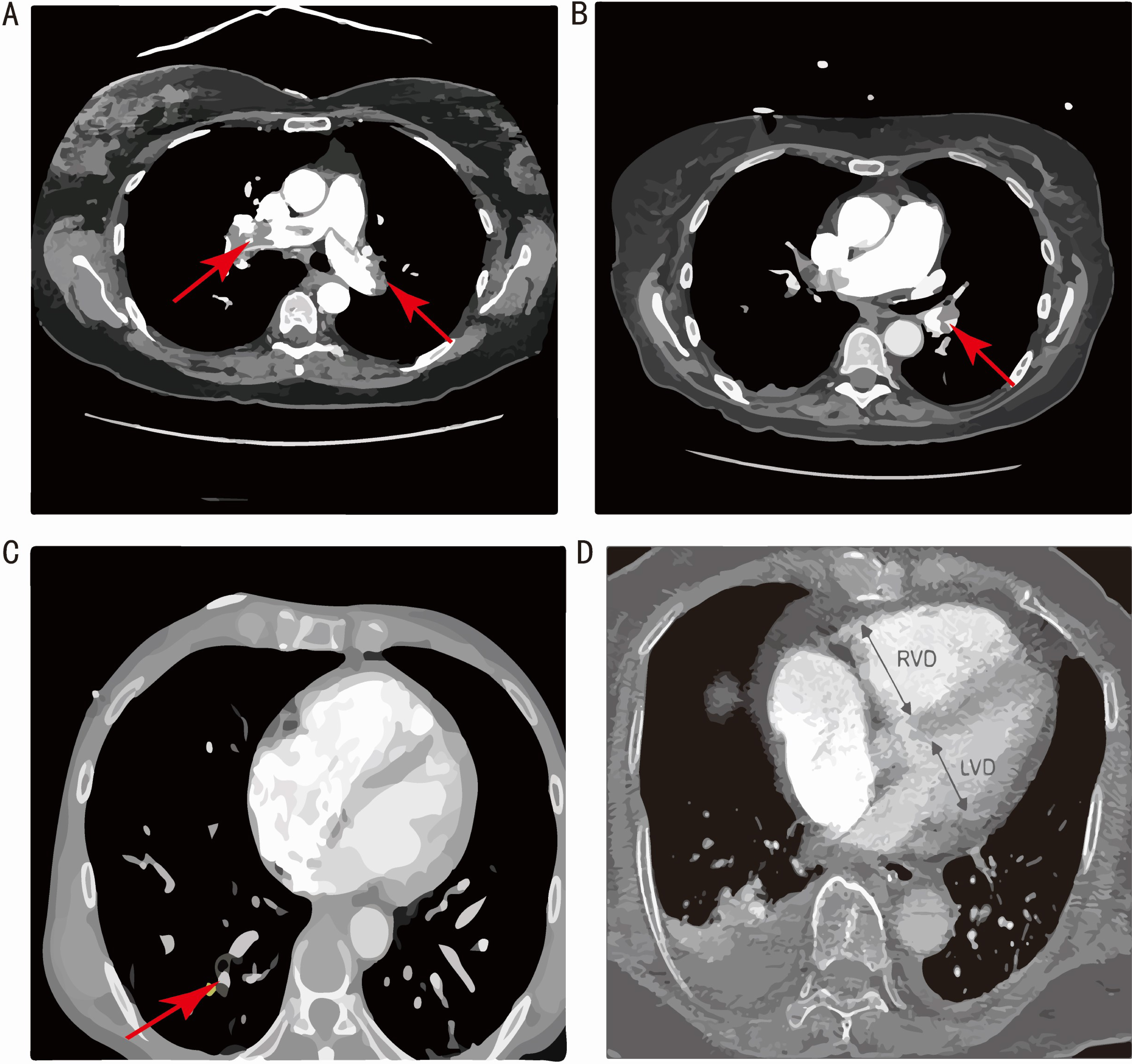
Background: Various electrocardiographic (ECG) abnormalities are associated with the severity of pulmonary thromboembolism (PTE). The utility of evaluating the clot burden of PTE based on ECG findings alone has yet to be thoroughly investigated in Chinese patients. The aim of this study was therefore to use ECG signs to establish novel models for quantitative and localization analysis of clot burden in patients with acute PTE. Methods: Acute PTE patients from three centers were enrolled between 2015 and 2019 in a retrospective cohort study (NCT03802929). We analyzed the 12-lead ECGs at admission and studied computed tomography pulmonary angiography (CTPA) features to obtain the Qanadli score of clot burden and location of thrombus. Novel risk prediction models were developed and validated using derivation and external validation cohorts, respectively. Results: A total of 341 acute PTE patients were screened, of whom 246 (72.1%) were from Shanghai Tenth People’s Hospital, 71 (20.8%) were from Shanghai Pulmonary Hospital and 24 (7.0%) were from Qidong People’s Hospital. In the derivation cohort, predictors included in the final models were congestive heart failure, chronic obstructive pulmonary disease, hypertension, coronary heart disease, atrial fibrillation and ECG abnormalities. The CHARIS (COPD/CHF/CHD, HTN, Atrial arrhythmias/AF, RBBB/RAD, Inverted T wave and S1Q3T3/ Sinus tachycardia) I model was established for quantitatively assessing Qanadli score. It had moderate discrimination in both the derivation cohort (concordance index (c-index) of 0.720, 95% CI 0.655–0.780) and the validation cohort (c-index of 0.663, 95% CI 0.559–0.757). The CHARIS II model was used to predict the probability of trunk obstruction. It showed similar discrimination in the derivation cohort (c-index of 0.753, 95% CI 0.691–0.811) and in the validation cohort (c-index of 0.741, 95% CI 0.641–0.827). Calibration curves and Hosmer-Lemeshow test confirmed the accuracy of the risk prediction equations in the external validation dataset. Decision curve analysis showed the CHARIS I and CHARIS II algorithms had positive net benefits in both the derivation and validation cohorts. Conclusions: From quantitative and localization perspectives, the CHARIS algorithms can identify acute PTE patients with heavy thrombus burdens prior to imaging diagnosis. Clinical Trial Registration: NCT03802929, https://www.clinicaltrials.gov/study/NCT03802929.